Lateral Flow Assay Products & Services
Whether you are a global IVD company or a virtual start-up, developing and commercializing a lateral flow assay can present many challenges. Maybe you’re not able to achieve the clinically relevant range of your analyte, require extra manufacturing capacity to meet increased demand, or have a concept for an assay but need a trusted, experienced partner to make it a reality.
You’ve come to the right place.
We are a full-service lateral flow development company specializing in the manufacture of ultra-sensitive quantitative diagnostic assays. Our services span all phases of the development cycle from feasibility to manufacturing. We also offer industry-leading technologies for increasing assay sensitivity as well as starter kits to help you build and optimize your lateral flow assay.
Read on to see how our team can help!
Contact Our Team
End-to-End Lateral Flow Solutions – From Development Through Commercialization
At Fortis Life Sciences®, we can provide you with manufacturing assistance from the initial feasibility phase, right up to the final commercialization stage. This is how we do it.
Feasibility
We assist right from the very start; collaborating with you to define product requirements, to choosing reagents to engineering your proof-of-concept assay.
Optimization
Once you have your experiment designed, we can look at assay optimization and sample preparation stages.
Validation
Once the previous steps have been completed, we take the development through to the validation phase, to ensure the scale-up process is effective before commercialization of the manufactured product.

Carboxyl Gold Nanospheres
Formulated for attachment of a molecular recognition element
Starting at $150
Why Partner with Us

Development of competitive and sandwich assay formats for protein hormones, steroid hormones, protein/protein complexes, peptide, serological response (IgM and IgG antibodies), drugs of abuse, fungal infection, bacteria, and viruses

Integration with multiple reader technologies for rapid, mobile, and quantitative point of care applications

Over 15 years of manufacturing and optimizing colloidal gold, gold reagents, and robust, reproducible conjugates

Proprietary gold nanoshells for the most sensitive quantitative and semi-quantitative visual detection, eliminating the need for costly filters or lenses

ISO 13485:2016 Certified* and contract manufacturer of prototype lots with up to 50,000 test strips and scaled manufacturing partners worldwide for larger quantities

On-site manufacturing of colloidal gold and gold nanoshells ensure a robust supply chain and enable large-scale production of strips and conjugates
High-Volume Lateral Flow Assay Manufacturing
With our high-throughput lateral flow assay manufacturing capabilities, Fortis Life Sciences is the efficient solution to help bring your company’s completed assays to market.
We’ve combined our nanomaterials expertise and comprehensive knowledge of medical devices to develop innovative manufacturing solutions that are tailored to meet your unique needs. Our automated reel-to-reel reagent deposition and lamination platforms generate high-sensitivity devices with proven reproducibility under our ISO 13485:2016 certified Quality Management System*.
Our resident biochemistry experts and on-site commercial-scale automated manufacturing provide unparalleled flexibility from start to finish, helping medical device companies bring new products to market with ease.
*For products within the scope of our ISO certification.
Equipment & Expertise:
- BioDot reel-to-reel dispensing & lamination
- Low humidity dry room for lamination & assembly
- 3000 sq ft available for manufacturing
- Equipped to produce up to 10 million lateral flow tests each month
What Our Customers Are Saying About Us
See what some of our past customers have had to say about working with us:
Wellness Ready
Working with nanoComposix has been equivalent to having an entire outsourced R&D department rather than working with a CRO. In addition to performing lateral flow assay development internally, nanoComposix has recommended and overseen ancillary research by third party CROs to meet the objective of our assay development program. The entire nanoComposix team has consistently exceeded my expectations with regards to both research and development and planning for commercialization of the resulting diagnostic technology. I would highly recommend that any business strongly consider nanoComposix for lateral flow assay development.
Patrick Lawless PhD - President and Founder - Wellness Ready
Noonan Holdings
After evaluating numerous labs, I spoke with nanoComposix and their enthusiasm and expertise pushed them to the top of the list. Their critical thinking and ingenuity led to a solution in a dramatically less time than was expected. Their team was very collaborative, providing regular updates and an open line of communication throughout. I would recommend them to anyone looking for a partner in assay development.
Ben Noonan - President & Founder - Noonan Holdings
Real-Time Analyzers
We recently contracted nanoComposix to develop a proof-of-concept lateral flow assay for therapeutic drug monitoring. nanoComposix worked on the project as if it was their own and not a contract R&D project, which showed their commitment and enthusiasm. We were thoroughly impressed with their meticulous approach in planning, designing and executing the project. Weekly updates were provided to ensure that the project was progressing within the prescribed time frame. The team at nanoComposix demonstrated a very high degree of scientific knowledge and technical expertise to successfully develop a working assay for us. We will definitely use nanoComposix lateral flow assay development services for all our future needs.
Chetan Shende - Research Chemist - Real Time Analyzers
Lateral Flow Services Case Studies
See real case studies on clients we have helped and the projects that needed completing:
CLIENT: FORTUNE 500 PACKAGING COMPANY
The existing standard method of detection for this food safety assay requires multiple days and a skilled scientist to perform the test. Using ultra-sensitive nanoparticle technologies, nanoComposix is developing a rapid lateral flow assay that can identify dangerous bacterial pathogens in less than 10 minutes.
CLIENT: NON-PROFIT ORGANIZATION DEVELOPING ASSAYS FOR DETECTION OF NEGLECTED TROPICAL DISEASES
Using the gold nanoshells, the non-profit group was able to achieve the desired sensitivity for their assay. The assay was transferred to nanoComposix for transfer to scale-up manufacturing under our Quality Management System. The assays were manufactured (50K strips) for testing in third world countries and are currently being evaluated in the field.
CLIENT: MARKET LEADER IN VETERINARY DIAGNOSTICS
This customer turned to nanoComposix after three years of assay development where assay sensitivity could not be achieved by an internal team or with two separate contract assay development manufactures. The first company spent 6-months optimizing the assay and could not reach the required limit of detection. The second development company used proprietary nanobeads which achieved sensitivity but dried conjugate was unstable after 6 weeks. After a one-month effort, nanoComposix achieved the clinically relevant sensitivity using gold nanoshells.
CLIENT: DIAGNOSTIC DEVELOPER
Customer approached us to develop two immunoassays to quantify two biomarkers for a physiological disorder. Although commercial tests (e.g. ELISA) existed for the quantification of these biomarkers, the customer perceived a market need for a simple, fast, and inexpensive method of detection. The initial phase of work successfully identified optimal affinity reagents to create a novel immunoassay for each biomarker. The immunoassays were then used to assess the diagnostic performance of each biomarker in a panel of diseased and non-diseased saliva samples. Following the successful outcome of phase one, the customer engaged with us to develop a multiplexed lateral flow assay to qualitatively detect both biomarkers. This work included the initiation of design controls and the manufacturing of pilot lot of tests.
CLIENT: DIAGNOSTIC INSTRUMENT MANUFACTURER
We were approached to assist in developing a point-of-care lateral flow assay to monitor a therapeutic drug level in patient saliva. During the first phase of work, we successfully identified optimal affinity reagents and developed a conjugation method to attach antibodies with spectroscopic labels to BioReady 150 nm Carboxyl Gold Nanoshells, enabling analyte detection using the client’s spectroscopic platform. Based on a successful phase one, the customer contracted with us for phase two, where the assay will be optimized and design controls will be implemented to produce a prototype device.
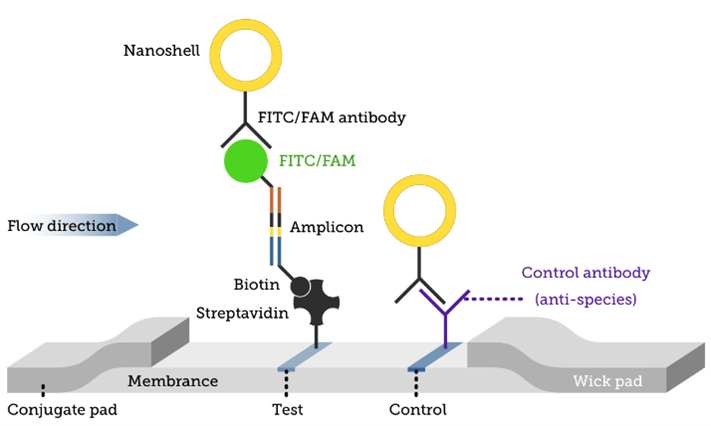
We developed and manufactured a lateral flow sandwich assay to detect amplified RNA or DNA fragments (amplicons) labeled with FITC/FAM and Biotin.
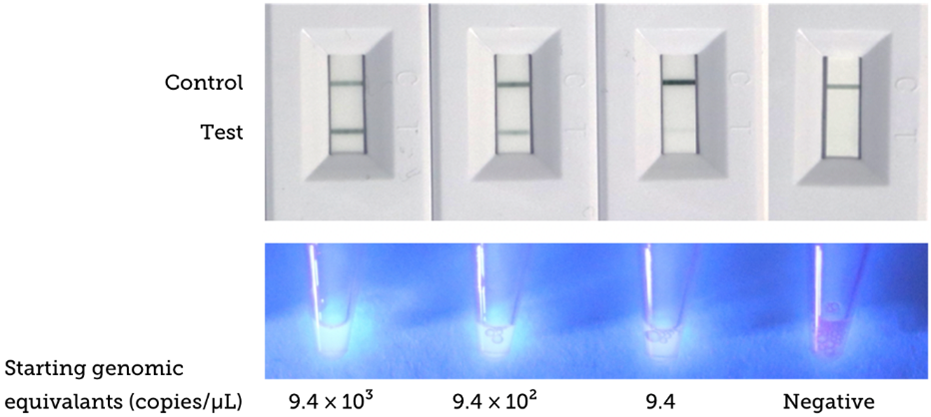
To help in the fight against COVID-19, this assay is being used to detect an amplified novel coronavirus (2019-nCoV) target RNA sequence from lysed, heat-inactivated virus samples at decreasing concentrations. Performance of the optimized LAMP-based assay and quantitative detection of the virus DNA product was examined using this device and verified with SYBR nucleic acid intercalating fluorescent dye. Both fluorescence and our assay results agree, confirming the functionality of the LAMP assay. This assay provided an adequate dose-response to enable the determination of quantitative Limit of Detection (LoD) and other key performance metrics. Image results of the dose-response experiment are presented below.
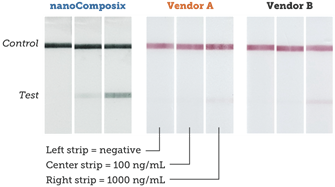
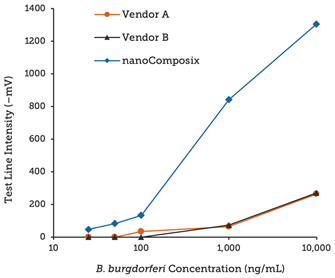
Using BioReady 150 nm Carboxyl Gold Nanoshells as reporter probes, we developed a rapid diagnostic to detect the presence of the Borrelia burgdorferi bacteria in ticks, which when transmitted by tick bites, can lead to Lyme disease.
Leveraging the unique optical properties of BioReady Gold Nanoshells along with nanoComposix’s assay development expertise, our lateral flow test strips provide 4× and 10× lower LOD (Limit of Detection) than similar test kits available on the market, as shown below.
Strips run with Borrelia antigen dilutions and read on a Qiagen benchtop reader.
Talk to Our Development Team
We’re here to help!
Our knowledgeable technical staff is available to help with your specific product, project, and technical questions. We look forward to learning more about what you’re doing and how we can help you succeed.
For custom materials, you can also check out our custom page to learn more about our custom capabilities and to submit a Custom Material Request Form to get started.
By clicking “Acknowledge”, you consent to our website's use of cookies to give you the most relevant experience by remembering your preferences and to analyze our website traffic.